MHRA should release the raw data for public scrutiny
The MHRA Yellow Card reporting system is designed to provide a signal of possible problems with new drugs based on reports of suspected adverse reactions from qualified medical practitioners. The data collected could be of much more value if more details were published. The MHRA shares such information with the pharmaceutical industry but, despite its role being to protect the public and relying on public funding, this data is not put into the public domain.
To make the most of what information is available the reports on different vaccine types can be compared. Any side effects that are a result of the production of the spike protein itself may be similar between all vaccine types. However, if one vaccine type has a much higher rate of a particular adverse effect than other vaccine types then this is suggestive of a genuine causal relationship. Confounders such as age may account for part of these differences, which is why publishing the raw data is so important.
Data sharing
The Yellow Card scheme is administered by the MHRA, a government body funded, at least in part, by the public. The data for the scheme is collected largely by NHS staff, who are again funded by the public. However, despite public finance being crucial to the generation of Yellow Card data, the MHRA have refused to release the anonymised individual patient data from this scheme for independent analysis (FOI 21/640). The MHRA argue that release of these data would be too onerous, yet paradoxically these same data are passed on to the vaccine manufacturers for analysis as a matter of routine (FOI 21/942). All that the public can access from Yellow Card is a rudimentary summary of the total numbers of adverse events recorded for each vaccine type in particular medical categories.
The MHRA’s attitude to data sharing stands in stark contrast to the situation in the USA, where the VAERS reporting system [2] provides anonymised individual patient data, and the detailed analyses that this allows has been crucial for recognising important safety signals [3] — albeit US Regulators have been slow off the mark in making full use of the data available to them. We note that the MHRA’s refusal to share the information that they hold within the Yellow Card database would not be tolerated in the general science community where access to raw data is now a prerequisite for publication in peer reviewed journals.
Despite the intransigence of the MHRA over the issue of releasing raw data from the Yellow Card scheme to the general public, it is incumbent upon the scientific community to make the maximum use of the data released from the scheme to scrutinise the validity of the conclusions that the MHRA reach in their weekly reports. This is particularly important to achieve because, despite FOI requests to see the scientific analyses on which their conclusions are based, the MHRA have been unable to produce any such reports (FOI 21/942).
Comparing frequency of reports by vaccine type
The weekly data released from the Yellow Card scheme takes the form of the total number of doses of each of the vaccines given, the total number of reports filed for each vaccine type, and the total number of adverse reactions recorded for each of a huge range of medical conditions compiled separately for each of the vaccine types. What insights can we gain from analysis of this information?
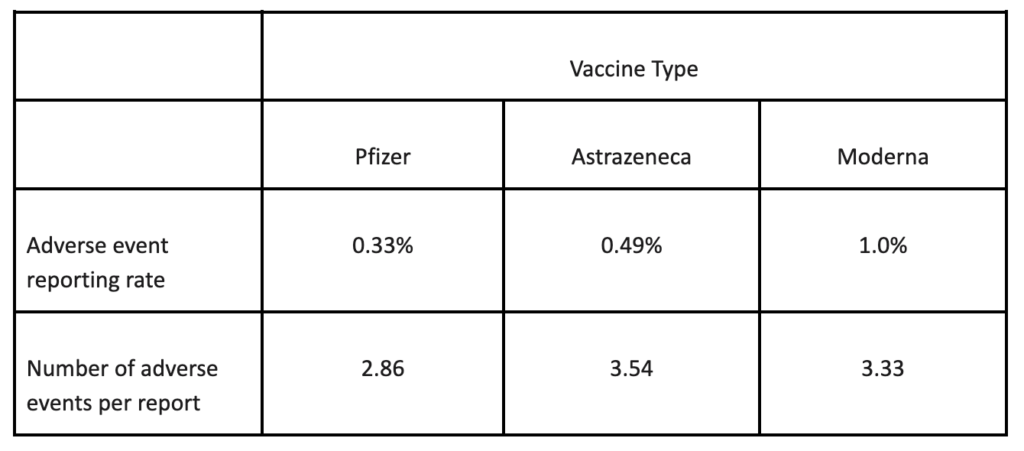
A simple question that we can ask is whether the different vaccines elicit the same or different rates of reporting of adverse reactions or number of reactions per report. The answer is clear (Table 1). There is something about a Moderna injection that generates a higher frequency of adverse event reports with less reactions per report than an Astrazeneca vaccination, which in turn generates a higher frequency of reports and more reactions per report than a Pfizer injection. The figures involved are so huge that these differences cannot be due to chance. There is something important happening that needs to be explained.
Risk of misinterpretation
Unfortunately, however, our interpretation can never be secure. The results we see could be due to the vaccines themselves. Alternatively, they could also be due to some confounding factor like the differences in age profile of the patients who were injected with different vaccine types, or to certain vaccine types being injected predominantly as boosters, or some combination of such factors. Yet distinguishing between alternative explanations is vital. If the effects we see are indeed due predominantly to vaccine type, this would have serious implications for vaccination policy and optimum choice of vaccine for minimising adverse reactions. However, analysis of confounding effects can only be achieved if the raw, anonymised individual patient data from the Yellow Card scheme are released by MHRA.
Comparing type of report by vaccine type
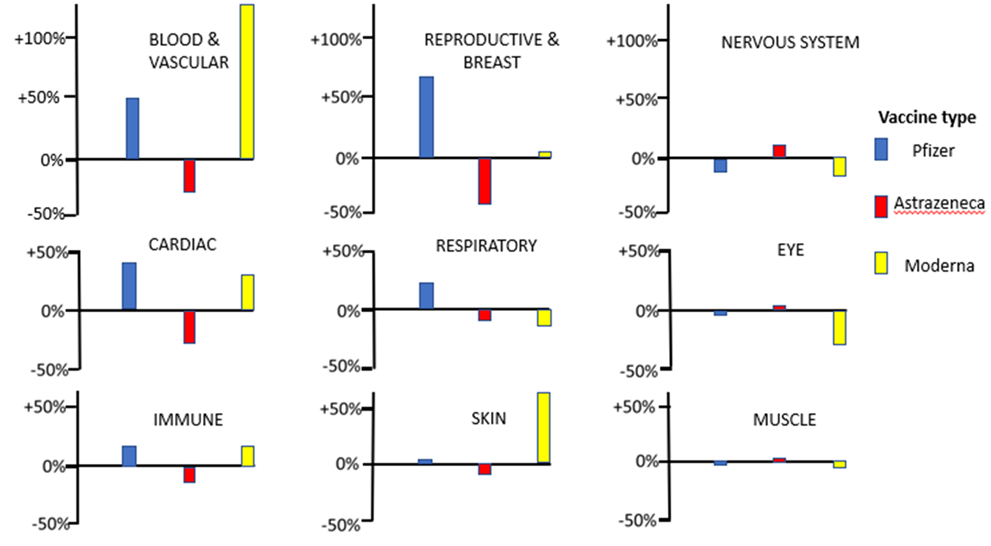
The second type of question that we can address using the Yellow Card data is whether choice of vaccines affects the spectrum of medical conditions recorded as adverse reactions. To answer this question, we can first sum up the number of adverse events elicited by each vaccine under the broad headings Blood & Vascular, Cardiac, Immune, Reproductive & Breast, Respiratory, Skin, Nervous System, Eye, Muscle and Other. A simple test for heterogeneity indicates that the relative frequency with which these classes of adverse reactions occur is highly dependent on the type of vaccine administered (χ2(18) = 29508, P<<0.001). Figure 1 illustrates the percentage by which the observed numbers of adverse reactions differ from the number expected if all vaccines elicited the same spectrum of adverse reactions. It is clear from the figure that departures from expectations are particularly large in the categories Blood & Vascular, Cardiac, Reproductive & Breast, and Skin; the different vaccines are eliciting quite different relative frequencies of adverse reaction in these categories.
For the categories Blood & Vascular, Cardiac, and to a lesser extent Immune and Reproductive & Breast, much higher than expected numbers of adverse reactions are elicited when the mRNA vaccines are administered, and lower than expected numbers of adverse reactions are found when the virus vectored Astrazeneca vaccine is used. Given that the same spike protein is encoded in the mRNA and virus vectored vaccines, this suggests that differences in the observed spectra of adverse reactions may be related to the mode of delivery of the spike encoding nucleic acid sequence in the vaccine. This observation for the Cardiac category is in agreement with a recent case series analysis which found that the risk of myocarditis is greater following sequential doses of mRNA vaccine than sequential doses of the adenovirus vaccine [4]. The role of the mRNA vaccine delivery system itself in eliciting adverse reactions must therefore come under scrutiny.
While this example shows that the Yellow Card data may be helpful for generating ideas and supporting other studies, the inadequacy of the partial information currently released by the MHRA means that our interpretation of such data will always be compromised. Again, we do not possess the means to control for possible confounding factors (age and sex of individual, vaccine dose number etc.) that could contribute to the results observed. Nevertheless, in this example, the sheer size of the apparent effects of vaccine type on the spectrum of adverse effects indicates that a thorough investigation is essential. If the vaccine effect were confirmed, this would have serious real-world implications for the Covid-19 vaccination programme and the safety and health of the UK population.
Conclusion
The data we need to carry out the necessary analysis to maximise the usefulness of the Yellow Card scheme has already been collected at the public expense and is currently held by the MHRA. We call upon the MHRA immediately to release the raw, anonymised, individual patient data from the Yellow Card reporting scheme to enable rigorous scrutiny of Covid-19 vaccine adverse events by doctors, researchers and the public. This echoes the recent call by BMJ editors for immediate release of raw data from trials conducted by vaccine manufacturers [5].
2. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html
3. https://jessicar.substack.com/p/a-report-on-myocarditis-adverse-events
4. https://www.medrxiv.org/content/10.1101/2021.12.23.21268276v1
5. Doshi P, Godlee F, Abbasi K. Covid-19 vaccines and treatments: we must have raw data, now BMJ 2022; 376 Covid-19 vaccines and treatments: we must have raw data, now | The BMJ
