Many of our readers will be aware of the claims of a rise in cancers since the vaccine rollout, particularly among the young. Last week we published an article discussing the scientific rationale why a rise in cancers might be expected after the vaccine rollout. The data published by the UK on this topic is contradictory but data from around the world is signifying there is a genuine problem.
Vaccine
Cancer concerns
The covid vaccines skipped safety testing for cancer risk. Pfizer said genotoxicity, carcinogenicity and biodistribution studies were “not considered necessary.” Even while their trial info sheet said “Due to the urgent need for a vaccine against Covid-19, with agreement from the MHRA, some of the tests usually required for a newly manufactured vaccine have been modified, in order to make the vaccine available more quickly for assessment.”

Have the MHRA admitted causation for any vaccine harm?
The MHRA are far too slow to react to harm and admit causation. There is a history here. When the 2009 swine flu vaccine caused narcolepsy the UK institutions were very slow to react. It was Scandinavian countries who highlighted the problem in 2010. That year the MHRA were measuring for other effects but not narcolepsy. In 2011, they said they needed to await epidemiological studies. It took until 2013 before Public Health England admitted a problem in the UK.

Medical Ethics have gone AWOL…again!!
Regular HART readers may remember last summer, we highlighted problems with a trial of a new Moderna mRNA booster which was recruiting in the US and the UK including healthy children from 12 years upwards. A letter was sent by the Children’s Covid Vaccines Advisory Council, signed by 125 health professionals and academics, to the CEOs and Chairmen of the 35 UK centres involved, pointing out the emotionally charged recruitment leaflet, the lack of compliance with Helsinki Agreement, and even the offer of money to children on trial completion, in contravention of research governance guidance.
When money and ethics collide
Last month, HART wrote an article on the spring booster programme, including linking to an article in Chemist+Druggist on the pending availability of private covid vaccines in pharmacies up and down the land. A company called Pharmadoctor was the first into the ring but has been followed by others including Boots, the oldest of the UK high street chemists, whose strapline on their website is: We serve your wellbeing for life.

The Smallpox vanishing Act
The primary beliefs of vaccinology are that it is possible to educate the immune system to protect against disease, that vaccines are safe and that smallpox was eradicated. It is quite possible to be very confident about the first points and still be able to question the evidence for the last one.

Vaccine injury support group get charitable status
UK CV Family is a peer-support group set up in 2021 by two vaccine-injured women, Charlet Crighton and Caroline Pover. Along with Vaccine Injured & Bereaved UK (VIBUK), they have faced an uphill battle in getting recognition. However, UK CV Family has recently been granted charitable status.
Where did the white clots come from?
Numerous funeral directors have now reported struggling to embalm bodies because of finding white “calamari-like” clots blocking the veins. What do we know about these clots? John Campbell has interviewed a series of funeral directors who describe a decreasing incidence of such clots but claim they remain in about 20% of those who die in 2023.

Top Cardiologist Calls on GMC To Investigate Covid-19 Vaccine Injuries
Dr Dean Patterson, a leading consultant cardiologist in Guernsey and Fellow of the Royal College of Physicians, wrote an extraordinary letter to the CEO of the General Medical Council (GMC) calling for an investigation into unprecedented harms from the COVID-19 vaccines.

The spring booster programme: now you need it, now you don’t
On 1st February, the website Chemist And Druggist announced the launch of private covid jabs, under the headline UK’s first private pharmacy COVID-19 vaccine service launched today.

New autopsy evidence from Japan: myocarditis – however mild – can result in fatal arrhythmias
The most concerning aspect of this is that the myocarditis itself was “focal and mild, as is mostly observed following COVID-19 mRNA vaccination” yet this resulted in a fatal arrhythmia, because cells which are part of the electrical system were affected.

MPs remain determined to go down with the ship
Dr Edmund Fordham is a HART member and a Prospective Parliamentary Candidate running against Lucy Frazer, MP for South East Cambridgeshire. Dr Fordham has sent us a letter that was shared with him by Lucy Frazer (his MP) discussing covid vaccines. It was written by Maria Caulfield, the Parliamentary Under Secretary of State for Mental Health and Women’s Health Strategy.
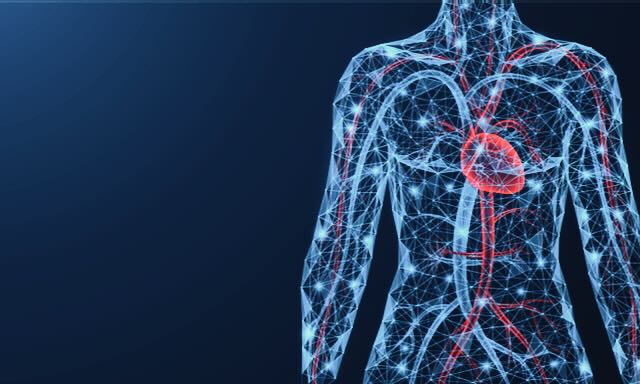
Cardiovascular effects of the covid vaccines
Covid vaccine harm is myriad and certain individuals have experienced a hugely debilitating syndrome of injury affecting more than one organ system. However, in terms of the sheer numbers affected the cardiovascular system is at the heart of the matter.

Who does ‘our’ NHS really serve?
The fact that the NHS is wasting millions of taxpayer pounds continuing to promote these ineffective and harmful products is really symbolic of what the health service has become. Simply another cog in the medical industrial machine, whirring to improve Pharma profits with little or no concern for end user health. In a service allegedly crushed by lack of resources, why on earth are they still pushing these toxic products on an unsuspecting ‘vulnerable’ sector of society?

Joint Open Letter to the Secretary of State
When will the Secretary of State order an investigation into vaccine harms? Excess deaths and increasing levels of sickness but still no transparent investigation.

Three years on from the first covid vaccine death
3 years ago today, Dr Stephen Wright died from vaccine-induced thrombosis, caused by the AstraZeneca covid vaccine he received on 16th January 2021.
Like many others he was assured it was Safe and Effective and that he should take it to protect others.
His family mentioned the vaccine could be the cause when he was hospitalised and a Yellow Card was completed in early February 2021. MHRA took zero action on this 1st known death!
Open letter to Chief Medical Officers 06-09-2021
Children do not need Covid vaccines 6th September 2021 6th September 2021 Open letter to: Professor Chris Whitty – CMO England: Email [email protected] Michael McBride – CMO Northern Ireland: [email protected] Gregor Smith – CMO Scotland: [email protected] Frank Atherton – CMO Wales: [email protected] Dear Professor Whitty, Dr McBride, Dr Smith and Dr Atherton, We are a […]
Open letter to the JCVI – 19th August 2021
Children do not need Covid vaccines Open letter to Joint Committee on Vaccination and Immunisation 19th August 2021 Dear Professor Lim and colleagues, As you know, a group of 60 British doctors and scientists wrote to the MHRA in May with a copy to members of the JCVI, expressing our concerns about the potential […]