
What took so long?
All HART articles also on Substack. Please consider a PAID SUBSCRIPTION so we can continue our work. Comments are open so you can join in the conversation.
The PRINCIPLE trial aimed to evaluate the effectiveness of ivermectin as an outpatient treatment for covid. The results were published in a much lower profile journal than published the results on other aspects of this trial. It has been over a year and a half since the trial finished. Other drugs included in the PRINCIPLE trial were reported on far faster. The delay and lack of press release both hint at the overall approach to ivermectin compared to less safe but far more commercially profitable drugs.
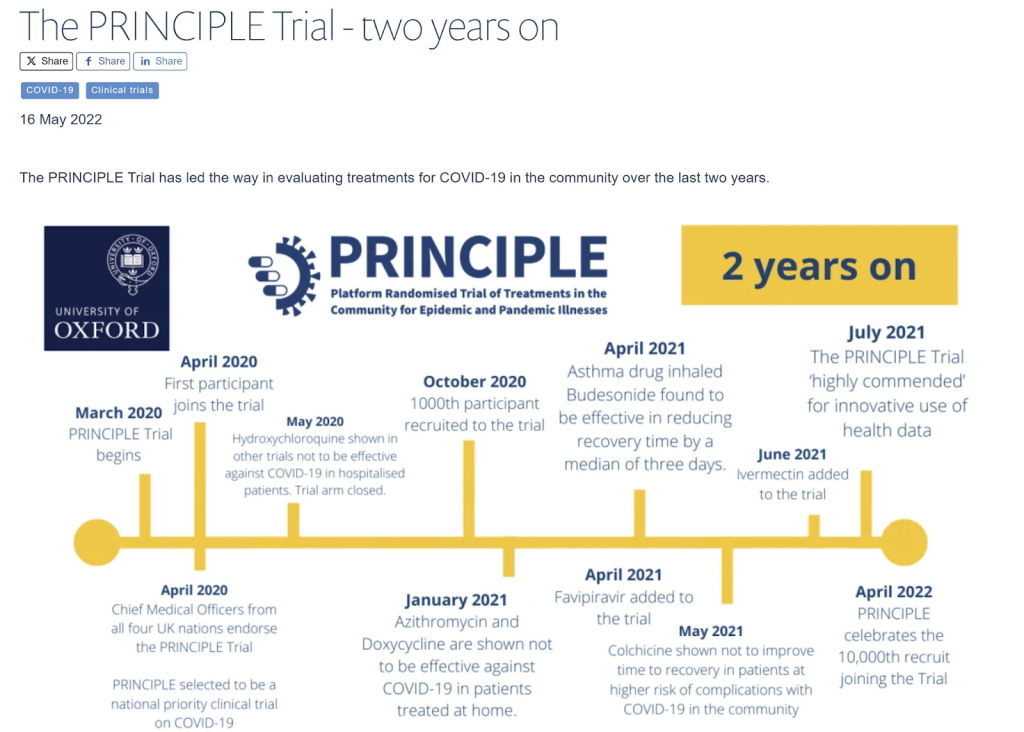
Both the World Council for Health and Dr. Pierre Kory have pointed out numerous flaws in the trial’s design and execution.
The trial’s negative interpretation of ivermectin’s benefits, despite evidence to the contrary, is seen as a deliberate attempt to undermine the drug’s potential efficacy. Kory argues that the data manipulation and presentation in the PRINCIPLE trial went beyond mere design flaws, constituting what he terms “research fraud.” He contrasts the trial’s handling of ivermectin with that of molnupiravir, noting a biased approach that favoured the latter. It is notable that Prof. Chris Butler was Principal Investigator on both trials which had overlapping recruitment but a notably different approach. The table below was put together by a group of PhD researchers and scientists.
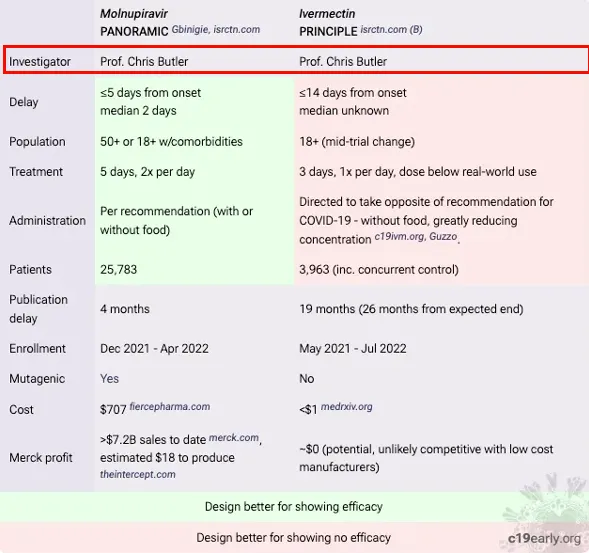
There were numerous ways that the trial was set up to fail. Patients were enrolled up to 14 days after symptom onset far too late to benefit from any antiviral effect. They were only treated for 3 days which is shorter than any normal antiviral protocol. They gave a low dose, only once per day. Furthermore it was recommended to be taken when fasting despite better absorption with food.
A serious trial would want to include people at risk of a negative outcome in order to demonstrate a benefit. However, the protocol was changed part way through to include any over 18 year old no matter how healthy and they removed the option for patients to collect the drug from a pharmacy, further delaying the treatment.
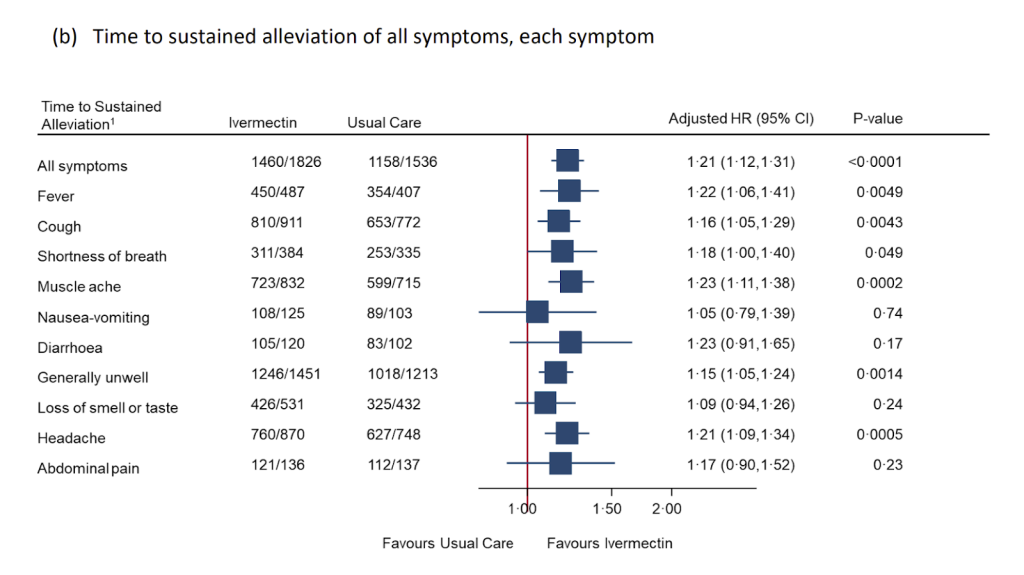
Even after all these obstacles to success they still couldn’t hide a statistically significant improvement in symptoms with a shortened time to recovery and a 36% reduction in people with “long covid” symptoms (a result that was hidden in the appendix). Reduced recovery times is highly correlated with a lower mortality in numerous trials (where they were designed in a way that could demonstrate this!). A rational response would have been to optimise the protocol to maximise potential benefit, rather than to dismiss the drug’s potential benefits outright.
There needs to be a reevaluation of research practices, emphasising the need for transparency, robust methodology, and independence from pharmaceutical industry influences. The PRINCIPLE trial’s saga is a case study in the complexities of conducting and interpreting medical research in an era where public trust is both more critical and more fragile than ever.
