
And you thought Covid vaccines for children were over!
You may think that Covid-19 vaccines for healthy children have been withdrawn but don’t worry your child can get their next fix by enrolling in the NextCOVE trial, launched last month in Bradford, with several other centres across the UK due to start recruiting soon. The trial was announced by Yahoo ironically on the same day, 30th June, that all routine covid vaccines ended.
The information leaflet opens with this emotionally loaded paragraph:
‘The COVID-19 pandemic is like nothing we’ve seen in more than a century and it has altered each and every one of our lives. Now, you or your child could be a part of important research on an investigational COVID-19 vaccine. By enrolling in the NextCOVE Study, you or your child will be contributing to a potential solution to the evolving COVID-19 pandemic, which has affected the entire world.’ and a suitably emotive picture below.

Further on it states: ‘By taking part in this trial, you or your child could make a difference for your family, your community and people everywhere.’
So what is this trial? NextCOVE is a trial comparing two types of Moderna mRNA vaccines for use as boosters: the original vaccine produced against the Wuhan strain, compared against the latest bivalent vaccine, which is a 50:50 mix of the original with a new omicron strain. Needless to say, no saline placebo is on offer; perhaps they regard it as unethical to have children missing out on the Magic Mixture!
It is notable that the only group currently able to get a covid vaccine are high risk children aged 6 months to 4 years, all other vaccines having been suspended through the summer. Boosters are likely to be available again this autumn for specified high risk groups. Healthy young adults, let alone children, are not recommended to have boosters.
It is hard therefore to see how these boosters could benefit the children entering the trial, an absolute requirement for children entering clinical trials. The Declaration of Helsinki, adopted in 1964, states that ‘All medical research involving human subjects must be preceded by careful assessment of predictable risks and burdens to the individuals and groups involved in the research in comparison with foreseeable benefits to them and to other individuals or groups affected by the condition under investigation.’
The Medicines for Human Use (Clinical Trials) Regulations 2004 enacted the Declaration of Helsinki into UK law, and further provides that ‘The rights, safety, and well-being of the trial subjects are the most important considerations and shall prevail over interests of science and society’.
The landmark international Universal Declaration on Bioethics and Human Rights (2005) states that, ‘Scientific research should only be carried out with the prior, free, express and informed consent of the person concerned”. Article 7, referring to people without the capacity to consent, states, ‘authorization for research and medical practice should be obtained in accordance with the best interest of the person concerned” and that “research should only be carried out for his or her direct health benefit’.
The trial information leaflet does not mention any direct benefit to the recipients, the aim of the study being ‘to learn more about how it works in the body’. The JCVI recently published a table of numbers needed to vaccinate and for the age group 16-19, it requires 193,500 vaccinations to prevent one severe hospital admission. For healthy 20-29 year-olds the number is even larger at 418,100.
But if there is no benefit, what about potential harms? In fairness, if you read through to the last page of the information leaflet, you can find:
- ‘The trial vaccine is investigational and has not yet been approved for use in adults or children. This means we are still researching the product and we do not know if it is effective and safe to use. We do not know if it will prevent SARS-CoV-2 infection or reduce the severity of COVID-19 illness.’ (surely this sentence alone precludes children entering the trial!)
- All vaccines may have some side effects and this is true for this investigational vaccine too.
- In other trials of individuals receiving investigational vaccines similar to mRNA-1283.222, the most common side effects were fever, headache, muscle aches or pain, joint aches or pain, tiredness, nausea/vomiting and chills. These side effects have been more commonly reported after the later injections of the investigational vaccine and typically last between 2 and 3 days.
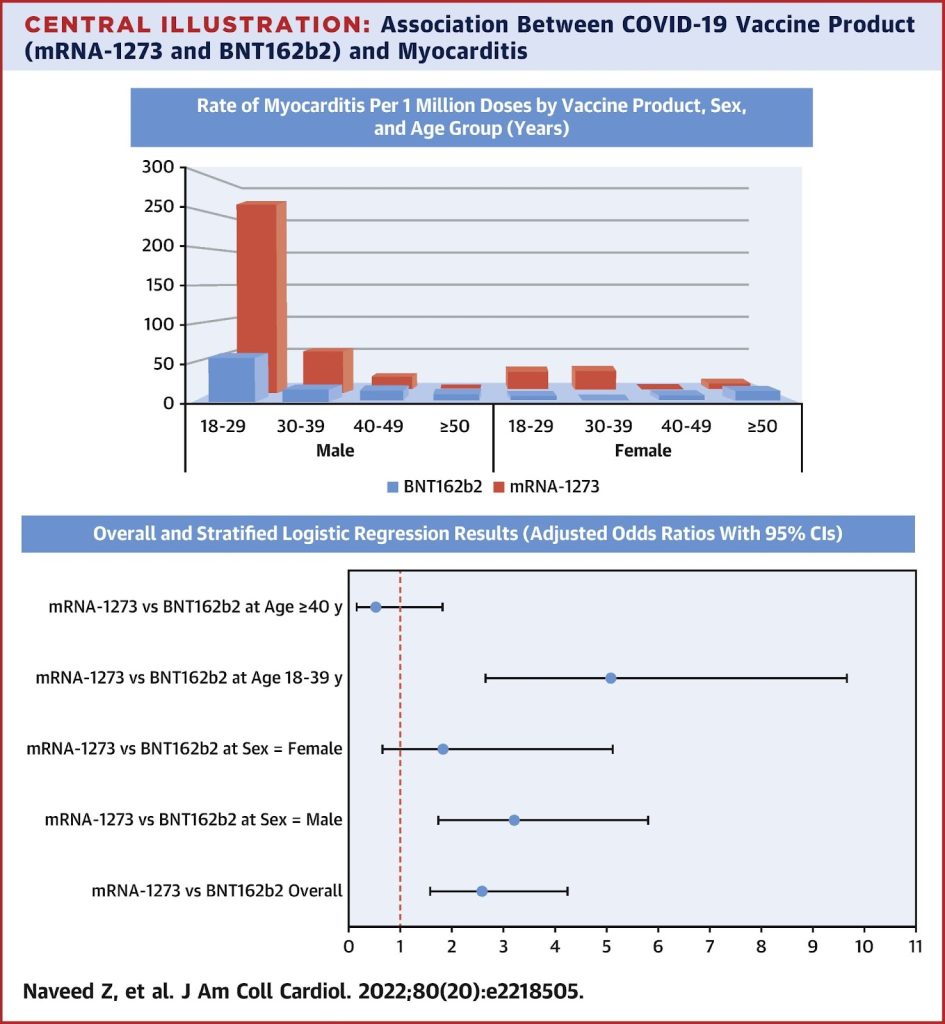
There is no mention of myocarditis in the information leaflet, despite the fact that the highest age group for this serious complication is 16-17s according to information presented to the FDA at 69.1 cases per million second doses in males. A further paper from the same group gave a rate of 105.9 per million doses. These studies are based on passive reporting systems which may underestimate the numbers affected. In another study from Thailand in which boys were brought back for blood tests on day 3 and 7 after their second dose, clinical or possible subclinical myocarditis/pericarditis was found at an extremely worrying rate of 1 in 43 of those injected. Many reports only include the Pfizer vaccine which was the predominant mRNA vaccine in use, but a study from Canada shows the incidence of myocarditis after Moderna was significantly higher than after the Pfizer vaccine, especially in young adults aged 18-29, as shown below.
There is also no mention that far from side effects ‘typically lasting between 2 and 3 days’, the effects of myocarditis may include permanent scarring to heart muscle with increased risk for sudden death. It has been suggested that post-vaccination myocarditis in children is mild and settles quickly, but this is not borne out by the facts.
One small follow-up study has been published in which 69% of the children studied still had significant abnormalities on cardiac MRI scans 3-8 months after their initial illness. The Late Gadolinium Enhancement (LGE) seen on the scans has previously been identified as an indicator of risk for later deaths. In a large follow-up study from the US, 519 children with myocarditis reported to the VAERS system, were followed 3-6 months later. 30% still had intermittent pain, 26% were still on medication. Of 151 who had a follow-up cardiac MRI scan, 54% were still abnormal. There has been sufficient concern that the FDA is now funding a large study of children with MACiV: Myocarditis After COViD Vaccination.
An analysis of the results from the original Pfizer and Moderna mRNA vaccine trials found that 1 in 800 suffered a “serious adverse event of special interest”, as defined by the WHO-endorsed Brighton Collaboration. Thus on balance, the risks from these trial injections potentially outweigh the benefits by as much as 500:1. Indeed, the JCVI itself concluded in July 2021 that the balance of benefit and risk was too close to recommend to healthy under 18s – and that was long before omicron, and at a time when we knew a lot less about the poor efficacy and apparent immune imprinting of the vaccines. Maybe the organisers of this trial will try to quote this new BMJ article on hospitalisations in children in England, but they might want to read a different analysis which has looked at the data included in the supplement and reached a very different conclusion. HART has also critiqued this article and found huge gaps.
Appeals for participants have been appearing in the press. Even more shockingly, another team from a London teaching hospital are advertising on WhatsApp, including the following quote:
What’s in it for them?
£1500 on completion of the study
There have to be question marks over whether this is even legal. It certainly contravenes the Ethical Research Involving Children (ERIC) guidance, which specifically advises: ‘Ensure that any payment is not used to unduly bribe, coerce or pressure children or parents to participate in research’
Any parents considering entering their children in this latest trial should cast their minds back to the AstraZeneca children’s trial. Oxford University started recruiting children in February 2021, despite the death the previous month of 32-year-old Stephen Wright, wrongly attributed to a co-incidental stroke. Correspondance then with the lead investigator was hardly reassuring. And less than a month later, deaths were being reported across Europe. The children’s trial was quietly suspended. The same research group and Research Ethics Committee have been called out by the Children’s Covid Vaccines Advisory Council on a booster trial in children a year ago.
Come back Nelson Mandela, who famously said: “There can be no keener revelation of a society’s soul than the way in which it treats its children” HART editors are aware we’ve used this quotation far too many times in the last 3 years – perhaps that is telling us something about the soul of our society, or at least of those who’ve sold their souls to Big Pharma.
A letter has been sent to the CEOs of all the participating centres to point out the potential breach of the Medicines for Human Use (Clinical Trials) Regulations 2004.
The first reply has been received from the Chief Medical Officer at Bradford Teaching Hospitals NHS Foundation Trust;
“Thank you for raising this issue. I have discussed with our Research Team, the Director of our Patient Recruitment Centre, and our Paediatric Team, and am satisfied that our processes are robust and fully encompass informed consent for this international study which has ethical approval. I would suggest that you contact Moderna, the trial sponsor, for further information.”
That is to say, an American drug company outranks a UK NHS Trust. Good to know!
Let’s hope parental instincts are more alive than the moral compasses of the Trust CEOs. £1,500 will seem like a paltry sum if your child loses this abhorrent game of Russian roulette.
